Propagating hope to healing through sustainable manufacturing
At Lupin, manufacturing is more than just a process — it is the cornerstone to upholding our commitment to patients. Every batch we produce carries with it our responsibility – the highest levels of quality, safety, sustainability, and access to trusted medicines globally. Our approach to quality is proactive, embedded, and non-negotiable. It is supported by robust systems that ensure consistency, transparency, and accountability at every stage of manufacturing. The pursuit of manufacturing excellence is deeply tied to our broader purpose of catalyzing treatments that transform hope into healing.
In FY25, we continued to strengthen this foundation by investing significantly in our API and formulation infrastructure. This is a testament to enhancing our efficiencies across the value chain - building smarter and agile systems that optimize efficiency, ensure regulatory excellence, and deepen supply resilience. By embedding advanced technologies across our operations, from sourcing and production to logistics, we are creating a futureready network that can respond swiftly to patient needs, regulatory expectations, and global market dynamics. These efforts don’t just help us meet standards — they empower us to set new benchmarks. They enable us to minimize risk, maximize efficiency, and maintain the highest levels of product integrity. For our customers, this means reliable access to high-quality medicines. For our shareholders, it means a resilient, high-performing operation that is built for sustainable growth.

15
Global
Manufacturing Sites
14
U.S. FDA
Approved Units
20,758 Mn
Total Formulation Units
Manufactured
>3,700 MT
API Quantity
Manufactured
Material Topics
World Class Sustainable Manufacturing Capabilities
Our global manufacturing network is the engine that powers Lupin’s commitment to high-quality, accessible healthcare. With 15 world-class facilities across three continents, we don’t just make medicines — we deliver trust, at scale. Every site is built to meet and exceed stringent national and international cGMP standards.
These facilities represent centers of excellence that blend technology, sustainability, and operational rigor.
By combining scale with precision and sustainability with performance, we are shaping a manufacturing ecosystem that is as resilient as it is responsible.
Units Manufactured
20,164 Mn units
Oral Solids50 Mn units
Oral Liquids1 Mn Units
Injectables10 Mn Units
Ophthalmic Liquids2.7 Mn Units
Topicals528 Mn Units
Inhalers (including dry powder and metered dose inhalers)Lupin is pioneering a digital-first, process-led transformation in pharmaceutical manufacturing, combining real-time data, AI, and advanced analytics to drive operational excellence.
 Digital and Data-Led Efficiency
Digital and Data-Led Efficiency
Harnessing IIoT and AI for predictive insights and smarter decisions.
 Process Innovation
Process Innovation
Advancing sustainability and efficiency through green chemistry and analytics.
 Operational Excellence
Operational Excellence
Fostering a culture of continuous improvement that is people-led and system-driven across our global network.
 Digital and Data Led Efficiency
Digital and Data Led Efficiency
Lupin is driving a digital-first manufacturing revolution by integrating real-time data and IOT (Internet of Things) in manufacturing, AI, and advanced analytics to enhance operational agility, product quality, productivity, and cross-functional performance through digitized monitoring and reporting.
Real-Time, Data-Driven Manufacturing
At Nagpur Unit-1 OSD and Tarapur, we introduced Industrial Internet of Things (IIoT) video walls. These systems capture and display real-time operational parameters across compression, fluid bed drying (FBD), granulation, and coating machines — enabling teams to monitor deviations, optimize processes, and model rapid Advanced Analytics (AA) interventions. At Tarapur, equipment sensors feed directly into the IIoT ecosystem, allowing for predictive analysis and timely course correction.
The deployment of PAS-X MES dashboards transformed how we track batch progress. With automatic data refreshes every three hours, teams now make sharper decisions with better prioritization — all through a single, unified digital interface.
Enhanced Line Visibility and Performance Monitoring
We digitized three out of four packaging lines at Nagpur Unit-1 OSD, enabling real-time tracking of hourly production rates. This not only empowers on-ground teams with actionable insights but ensures faster corrective responses. We plan to scale this visibility to Somerset.
In Tarapur, time cycle e-log dashboards now span four plants, allowing real-time tracking of critical batches. Similar dashboards have gone live in our sites in Dabhasa and Ankleshwar — supporting shift transitions, enabling product-level robustness analytics, and enhancing overall throughput without compromising quality.
Integrated Command and Control
Our newly introduced Digital Performance Management Dashboard integrates KPIs across six core functions — Finance, Supply Chain, HR, Manufacturing, EHS, and Quality — offering leadership a bird’s-eye view of performance trends, bottlenecks, and improvement areas. This dashboard acts as a single source of truth for driving cross-functional alignment.
Smarter Yields Through Advanced Analytics
AA modeling was deployed on compression parameters for five key products at Nagpur Unit-1 OSD, resulting in significant yield improvements. With proven results, we are now scaling this initiative across the product spectrum at the facility.
Together, these interventions represent not just digital progress — but a profound cultural shift. One where data, technology, and people come together to redefine how we deliver consistent quality, faster turnarounds, and sustained operational excellence.
AI and GenAI Transformation
At Lupin, we are embracing Artificial Intelligence (AI) and Generative AI (GenAI) to drive smarter, faster, and more efficient operations across the value chain. Our AI initiatives are reshaping how we work, from field operations to manufacturing and compliance.
Smart Manufacturing Hub: AI-enabled insights for querying critical process and maintenance data, improving operational agility.
Quality Co-Author: Automates and accelerates documentation like SOPs and QRAs, ensuring compliance and efficiency.
OOS Navigator: Streamlines investigations with AI-driven root cause analysis and auto-generated reports.
Supply Chain Insights Hub: Provides real-time responses to market signals and disruptions, enhancing planning and resilience.
 Process Innovations
Process Innovations
Driving Process Innovation by Integrating Green Chemistry Principles
At Lupin, we believe that process innovation is more than a pursuit of efficiency — it is an ethical imperative. We are reimagining manufacturing to balance business performance with environmental responsibility, embedding sustainability into the way we develop, refine, and scale every process. We have Process Development Laboratories at each of our manufacturing locations. These centers concentrate on refining processes to improve on new routes of synthesis, cost improvement processes, implement operations-friendly processes, and work on packaging development.
Our adoption of Green Chemistry principles reflects this approach. By prioritizing safer reagents, cleaner reactions, and smarter synthesis pathways, we aim to minimize waste at its source — not just manage it downstream. Metrics such as atom economy, E-factor, and Process Mass Intensity (PMI) guide us in selecting the most environmentally responsible routes.
In FY25, these principles translated into a meaningful outcome. For example, for a key molecule in the antidepressant class, we redesigned the resolution stage — optimizing raw material usage and improving selectivity. This change alone eliminated 57 metric tons of waste, significantly reducing the environmental footprint of a high-volume product without compromising yield or quality. This is just one example of how we embed the principles of green chemistry into our process development goals. We institutionalized this mindset by training our scientists across R&D and manufacturing on these principles, ensuring that sustainable design becomes the norm — not the exception.
Today, many of our development routes already demonstrate industry-leading sustainability metrics: E-factors under 100, improved atom economy, and lower PMI values. These initiatives reinforce our commitment to environmental protection.
For us, process innovation is not confined to cost or speed. It is about accountability — to regulators, to patients, and to future generations. Every optimization we undertake must uphold the highest standards of quality and compliance while advancing our collective responsibility to heal without harm.
Greener API Manufacturing Process
We redesigned the API (antidepressant category) production process, which previously relied on non-recoverable solvents and high-water usage. The new process helps in reduction of reaction steps and time, simplifies work-up, and delivers higher yields with exceptional purity. It also reduces impurities, including undesired isomers, and uses antioxidants for product stability.
Outcome: Output increased by 7%, water use dropped by 17%, and solvent use reduced by 33%. The process limits powder processing to the API stage, lowering costs. Incorporation of antioxidants prevents product degradation, ensuring high-quality output.
Safer and More Efficient Process Adoption
We replaced a multi-step process for manufacturing an API (Selective Cholesterol-Absorption Inhibitors). The replaced optimized process reduces organic/aqueous phase handling and simplifies production through telescoping of the process.
Outcome: Yield improved by 7%, waste decreased by 40%, water and solvent use dropped by 35%, and powder handling steps fell by 50%. The E-factor improved by 33% (from 5.86 to 3.96), marking a greener process.
More Efficient Redesigned Process Scale-Up
We transformed a 5-step process into a 3-step process for an API (Antidepressant-Class Drug). This process involved selective imine reduction with a metal catalyst to achieve a higher yield of a chirally pure product. This cuts batch testing, reduces solvent use by 40%, lowers water use by 30%, and decreases waste by 16%. The process mass index improves by 40%, and the E-factor drops from 16.5 to 7.96. Atom efficiency rises from 64% to 84%.
Outcome: Yield increased by 63%, raw material use reduced by 30%, and residue per kilogram of product decreased by 30%.
We are building a resilient, digitized, and customer-centric supply chain — one that not only meets evolving regulatory requirements but stays anchored in improving patient outcomes worldwide.
Christoph Funke, Chief Technical Operations Officer

Case Study
Seamless Technology Transfers Through Stage Gate Excellence
In pharmaceutical manufacturing, the ability to transfer technology — from R&D benches to commercial shop floors or across production sites — is foundational to scale, speed, and quality. Being at the forefront of leveraging digital solutions to serve patient needs, we have institutionalized a Stage Gate Mechanism to ensure every transfer is deliberate, disciplined, and designed for success.
This milestone-based framework introduces a series of structured checkpoints — or “gates” — where cross-functional teams critically review key deliverables before the process can advance to the next phase. These reviews ensure that technical, regulatory, and operational criteria are thoroughly met before progressing.
Each gate functions as a moment of scrutiny and alignment. Experts from R&D, Tech Transfer, Manufacturing, Quality, and Regulatory Affairs come together to validate feasibility, ensure compliance, and proactively identify potential risks. This collaborative evaluation process enhances predictability and mitigates risks of disruptions that could potentially delay production or compromise product integrity.
Our centralized tracking system and well-defined KPIs offer real-time visibility into transfer milestones. This not only enhances transparency and governance but also empowers teams to make timely decisions — whether to advance, reassess, or course-correct.
By embedding rigor at every stage, the stage gate approach minimizes costly rework, accelerates scale-up, and ensures that every product reaches patients efficiently and reliably. More than a process control tool, it is a mechanism that instills accountability, cross-functional alignment, and operational excellence into every transfer — all in service of delivering better health outcomes, faster.
Digitizing Quality at Source
This year, we deployed the IPQA centralized software platform across four sites — Chhatrapati Sambhajinagar (CSN), Mandideep, Pithampur Unit-1, and Goa. The platform provides a fully integrated, digital-first solution that automates in-process quality checks across compression, coating, and capsulefilling operations. Built to meet global regulatory standards like EU GMP Annex 11 and FDA 21 CFR Part 11, IPQA ensures real-time compliance and data integrity. Electronic reports are autogenerated and stored in a central repository, eliminating manual entries and reducing error rates to zero. The system seamlessly integrates with MES and e-BMR platforms, marking a significant step forward in digitizing quality assurance at source.
Every batch we manufacture carries a promise — of trust, quality, and healing. At Lupin, purpose meets precision, and global impact is an outcome of every action.
Rajendra Chunodkar, President — Manufacturing Operations
Other Impactful Process Improvement Projects
| Innovation | Description | Advantage | Outcome |
|---|---|---|---|
| Parallel Bulk Manufacturing | We implemented parallel bulk manufacturing in our MDI line in Jammu, resulting in a time saving of 2 hours per batch during the initial test campaign. | Bulk manufacturing enables cost efficiencies by lowering per-unit production costs and optimizing resource allocation, essential for maintaining competitive pricing. Additionally, it ensures a robust and consistent supply of medications to meet demand fluctuations, supporting reliable distribution and market presence. |
|
| Glass Media Filter | Glass media filters are used in place of self-cleaning filters at Lupin Biotech, to enhance the efficiency of raw water filtration. | Glass media filters provide enhanced filtration efficiency and improved water quality due to their angular shape and durable material, leading to longer media life and reduced maintenance costs compared to conventional filters. |
|
| Shift Shop Floor Supervisor | The concept of Shift Shop Floor Supervisor has been implemented at Lupin Biotech to regulate and control the operations as well as manpower. | This dynamic layout can lead to improved workflow, reduced downtime during changeovers, and allows quick adaptation to different manufacturing processes – thereby optimizing productivity and operational efficiency. |
|
We invest in purpose-driven science. Our work in biosimilars focuses on making cutting-edge biologics more accessible – transforming hope into healing for patients around the world.
Dr. Cyrus Karkaria, President — Biotech Business
Case Study
Driving Smarter Planning Through Kinaxis
Lupin has strengthened its supply chain capabilities by implementing Kinaxis, a specialized digital planning tool that enhances production scheduling and decisionmaking. The platform enables recipe creation, capacity mapping, and generates initial production commits while tracking key metrics like Requirements Vs. Commits (R Vs. C) and Commitments Vs. Actuals (C Vs. A). These insights have led to reduced cycle times, improved visibility, and more agile operations — supporting our mission to deliver timely, cost-effective medicines to patients worldwide.

 Operational Excellence
Operational Excellence
SOP Simplification and Harmonization
We simplified and harmonized key SOPs across our OSD formulation sites — spanning operations, PD labs, warehouses, and engineering services. The focus: reduce complexity, enhance clarity, and standardize processes across sites. These SOPs are now leaner, more intuitive, and better aligned to FDA, ICH, WHO, and EMA requirements. This effort significantly reduces human error risks, while ensuring site-to-site alignment and regulatory compliance. In FY26, we will extend this initiative to our API facilities and harmonize key SOPs for Quality, Operations, and Warehousing across both formulations and APIs.
Human Error Reduction at Lupin Biotech
At Lupin Biotech, we launched a structured human error reduction program, ‘Procedural and Human Error Reduction Protocol.’ It includes SOP identification, resource mapping, Gemba-based gap detection, and visual flowcharts for SOP key points. The approach fosters greater ownership among shopfloor teams, prevents avoidable deviations, and strengthens procedural compliance.
Capacity Expansion for Future Readiness
We expanded manufacturing infrastructure across key sites in order to strengthen our capacity. At our Tarapur facility, we have increased the intermediate product manufacturing capacity from 5 TPM to 12 TPM and one intermediate MPP5 plant upgraded to meet the cGMP requirements and made it dedicated for single product. Our Goa facility now houses a dedicated Microbiology Lab; LRP-Pune facility is equipped with a new Bioanalytical Lab; and our Pithampur HPD facility has been scaled to support larger volumes. At our Nagpur site, we expanded the injectables plant by adding an autoclave, cold rooms, clean storage areas, and increased rack capacity.
Recognition for Manufacturing Competitiveness
It is a matter of great pride for us that our Ankleshwar API and CSN formulation facilities received Gold Medals at the 11th National Awards for Manufacturing Competitiveness (NAMC 2024–25). The award recognized our non-conventional approaches, custom-fit strategies, and site-specific execution, rating us on ten parameters that define long-term manufacturing competitiveness. This honor reinforces our commitment to operational excellence, strategic agility, and delivering measurable value across the supply chain.
Sustainable Manufacturing
At Lupin, we believe that true healthcare progress must go hand-in-hand with environmental stewardship. Our manufacturing philosophy is anchored in sustainability — ensuring that every product we make contributes not just to individual health, but also to planetary well-being.
Environmental stewardship is part of our core. In FY25, we continued transitioning our operations to renewable energy, while optimizing resource consumption across our manufacturing network. From adopting energy-efficient lighting, advanced pumping systems, and sustainable cooling technologies to investing in low-carbon processes, every action was purpose-led to enhance ecosystem resilience.
All our Indian manufacturing and R&D sites, along with our Mumbai headquarters, are ISO 14001 and ISO 45001 certified while our international sites undergo rigorous internal assessments reaffirming our compliance with global environmental and occupational health and safety standards. These certifications reflect more than procedural rigor; they signal a deep and enduring commitment to responsible operations.
Our investments this year in renewable energy and decarbonization are a step toward long-term climate resilience — a reiteration of our culture, to always be sustainable, inclusive, and future-ready.
Our Tarapur API facility earned a Gold Award at the 10th India Green Manufacturing Challenge (IGMC), organized by the International Research Institute for Manufacturing (IRIM). Additionally, our Ankleshwar site successfully completed the GMEA assessment in May 2024. The site was recognized as a “Leader” and “Sustainability Front Runner,” by GMEA Assessment auditors.
Product Quality and Safety
Our quality management systems continue to serve as the guiding hand to our manufacturing processes. This year we have implemented newer assessment models and enhanced our quality management practices to ensure that the medication we produce is of the highest standards. Lupin has 16 laboratories out of 21, certified at 5S level across our regulated sites. We advanced technology solution implementation in our labs successfully. Lupin Goa won the technology leader of the year award at the Manufacturing Today Conference and Awards-2024 for its innovative automated Mobile phase preparation assembly project. We have also developed a methodology to monitor and improve HPLC utilization, achieving cost savings equivalent to 52 new HPLC.
Across our organization, quality is a deeply embedded discipline that represents trust. Our Global Quality Management System (QMS) integrates best practices across our global network, creating a unified ecosystem led by more than 2,700 dedicated quality professionals.
Our governance structure ensures rigorous oversight through three key layers: Site Quality Councils, Quality Council Meetings, and the Global Quality Council Steering Committee. To further elevate this framework, we introduced the role of Chief Quality Officer — reinforcing accountability and uniformity at the highest level.
This year, we took decisive steps to benchmark and elevate our quality maturity. In alignment with the U.S. FDA’s Quality Management Maturity (QMM) initiative, we initiated structured self-assessments across six practice areas at our Tarapur API, Pithampur Unit 1 (API and Formulations), and Pithampur Unit 3 sites. These assessments were followed by on-site evaluations conducted by consultants who have worked on the FDA’s own QMM pilot programs.
At Lupin, quality is more than a standard — it’s our shared discipline and collective conscience. This unwavering commitment safeguards patients and builds trust worldwide.
Dr. Ranjana Pathak, Chief Quality Officer
The six focus areas included:
- Advanced Pharmaceutical Quality System
- Business Continuity
- Employee Engagement and Empowerment
- Management Commitment to Quality
- Technical Excellence
- Corporate Responsibility
We are preparing to volunteer for future FDA-led QMM pilots, with an intent to institutionalize best-in-class practices across our manufacturing sites. Insights from these assessments will guide systemic QMM enhancements, reinforcing quality as a strategic differentiator.
Our Process Development and R&D teams play a pivotal role in ensuring manufacturing integrity. From controlling input material attributes to defining product specifications and conducting rigorous end-product testing, every step is designed to safeguard quality. This disciplined approach — centered on critical quality attributes and process consistency — enables us to prevent deviations and maintain sigma levels above 4.0, ensuring our products reach patients with uncompromised quality and safety.



Regulatory Compliance
We remain steadfast in our dedication to regulatory compliance, ensuring adherence to benchmarks across all regions of our operations. We have made substantial progress this financial cycle and continue to monitor critical Key Performance Indicators (KPIs). These metrics serve as a compass, guiding our efforts and ensuring we stay on track to meet and exceed regulatory expectations. This proactive approach not only safeguards our operations, but also reinforces our position as a leader in pharmaceutical quality and safety.
| FDA Inspections | FY23 | FY24 | FY25 |
|---|---|---|---|
| U.S. FDA inspection (including GMP and Bioresearch center) | 9 | 3 | 6 |
| No. of Form 483 | 7 | 2 | 3 |
| No. of total observations | 55 | 3 | 17 |
| U.S. FDA inspection (including GMP and Bioresearch center) warning letters | 1 | 1 | 0 |
| Recall Type | FY23 | FY24 | FY25 |
|---|---|---|---|
| Class 1 recalls | 0 | 1 | 0 |
| Class 2 recalls | 7 | 6 | 9 |
| Class 3 recalls | 0 | 1 | 3 |
| Recall Type | FY23 | FY24 | FY25 | Target |
|---|---|---|---|---|
| Corporate internal audit on Lupin sites | 16 | 17 | 20 | Driven by Lupin’s internal quality metrics. All India sites are to be audited at least once a year by an internal audit team to ensure compliance and audit preparedness. |
| Total-supplier quality audits (third party finished product site) for India markets | 74 | 64 | 58 | Driven by supplier quality metrics, every supplier site is audited once in 3 years and at the time of new vendor qualification. |
| Total-supplier quality audits (third party finished product site) for U.S. markets | 6 | 3 | 2 | |
| Total-supplier quality audits (raw materials) for all markets | 250 | 265 | 167 | |
| Training for Employees – covering Quality Management System, Data Integrity SOP, and Annual cGMP | 6,385 | 10,090 | 8,057 | 100% of applicable employees |
Combating Counterfeit Medicines
Ensuring patient safety begins with preserving the integrity of every product we manufacture. At Lupin, we are unwavering in our efforts to prevent counterfeit medicines. Our multi-layered protection strategy includes tamper-evident packaging, serialization with unique identifiers, and scannable QR codes to verify authenticity. Every shipment is tracked through a secure transportation monitoring system, supported by detailed chainof- custody documentation. These safeguards uphold trust across the value chain — from our manufacturing sites to the hands of patients.
| Implemented Feature | Details |
|---|---|
| QR Codes | QR code implementation in top 300 products |
| Glue Packaging | Enhance the security of secondary packaging for EU markets |
| UV-Coated Foils | Top-selling brands, advanced security |
| Holographic Foils | Top-selling brands, advanced security, distinct 3D visual effects, challenging to duplicate |
| Multi-Color Security Print | Select high value/volume products |
Pharmacovigilance
Lupin’s Drug Safety and Risk Management (DSRM) team, responsible for pharmacovigilance, has implemented a comprehensive quality management system that adheres to global regulatory standards. The DSRM team has established accessible channels for consumers and healthcare professionals to contact Lupin regarding product inquiries or to report safety, quality concerns, and defective products. These include a dedicated call centre and email facility. Contact details are prominently displayed on product packaging and Lupin’s official website. Any product quality complaint is promptly forwarded to the manufacturing teams for thorough evaluation and investigation. The DSRM team meticulously reviews each reported adverse event, processing them in a validated safety database. Adverse events meeting specific reporting criteria are submitted to the relevant health authorities in compliance with local regulations.
Furthermore, the DSRM team proactively monitors product safety through comprehensive worldwide literature searches, periodic risk-benefit assessments, and rigorous signal detection activities. Key performance indicators, including compliance with submission deadlines for expedited and periodic reports to regulatory authorities such as the U.S. FDA, TGA Australia, Health Canada, Drug Controller General of India, and U.K. MHRA, are closely tracked. These KPIs undergo monthly reviews and are presented during Global Quality Council Steering Committee meetings, ensuring robust governance over Lupin’s regulatory obligations. This systematic approach underscores Lupin’s commitment to maintaining the highest standards of pharmacovigilance and product safety.
Manufacturing Division Upskilling and Development
At Lupin, we believe that continuous learning is the cornerstone of operational excellence and innovation. Last year, we launched the Parenteral Drug Associations Education Course. Taking this forward this year, we have launched the Lupin Training Academy at Nagpur for our employees across the network. The academy will be equipped with classrooms and practical training facilities with a dedicated content creation team and faculty with technical capabilities of Augmented Reality and Virtual Reality. The trainings will enable the employees with the required knowledge, skill set and competence for improving Right First Time Performance, and ensuring product quality, patient safety, and compliance. Our training and development programs are designed to build a future-ready workforce by fostering technical expertise, cross-functional collaboration, and a culture of improvement across all sites.
Gemba Walkthrough Program: Institutionalized across all facilities to promote real-time observation, cross-functional engagement, and proactive problem-solving. Monthly walkthroughs and recognition programs like ‘Gemba Stars’ and ‘Gemba Gurus’ reinforce accountability and continuous improvement.
All-Time Inspection Readiness: We launched an ongoing inspection readiness program at Tarapur and Mandideep Unit-1, focusing on critical quality subsystems. Supported by internal SMEs and external GMP consultants, the initiative includes subsystem evaluations, targeted workshops, and personalized training to prepare teams for inspections. Plans are in place to expand the program across additional sites and standardize best practices company-wide.
Ninja-X Gamified Learning Platform: With over 3,000 users and 75 AI-enhanced modules, Ninja-X fosters interactive learning and operational proficiency. Its expansion to global sites like Somerset (U.S.) and MedQuimica (Brazil) promotes consistency and cross-site collaboration.

Other Training and Development Programs
Pithampur U-2 Ophthalmic, Nagpur Unit-2 Injectables
Continued Collaboration with Parenteral Drug Association (PDA) on Employee Skill Development and Training: In fiscal year 2024, we addressed all the essential principles and core concepts with our front-line staff. In fiscal year 2025, our training emphasis shifted to key subjects, case analyses, challenges, troubleshooting, and investigative processes. Looking ahead to fiscal year 2026, we intend to focus on advanced decisionmaking for sterility assurance experts, strategic frameworks and methodologies, an in-depth exploration of regulatory matters, evaluation of interventions, compliance with media fill standards, and sophisticated risk-based strategies for contamination control.
Lupin Sites and Corporate Team
Workshop on Quality Management Maturity and Supply Chain Resilience: In partnership with the PDA India Chapter, we organized a workshop and a conference. These were focused on Quality Management Maturity and Supply Chain Resilience. The sessions offered valuable insights into transforming quality practices and ensuring a resilient supply chain. The conference and workshop aided understanding of how to pinpoint and mitigate supply chain weaknesses before they affect product availability. The discussions emphasized that a strong quality culture and proactive management are key to driving continuous improvement and operational excellence. Success in achieving Quality Management Maturity depends on a combination of technical expertise, employee engagement, skill development, and an empowered workforce.
Goa
World Class Lab Practices Training programs for Analysts were organized to reduce lab incidents.
Mandideep
World Class Manufacturing (WCM) and 5S Training in FML Function was provided for our site employees.
Mandideep and Tarapur
English-speaking coaching was provided to employees by external faculty to enhance communication skills and vocabulary.
GMP Compliance Enhancement and Regulatory Readiness programs were conducted at the Mandideep and Tarapur sites, with a focus on developing Subject Matter Experts (SMEs). These sessions were led by external and international GMP consultants.
Biotech, Pune
The S.O.A.R. program (Synergy, Open Communication, Action, and Results) was launched to strengthen team building. The sessions are conducted off-site.
On-site training on Contamination Control Strategy was conducted by the Parexel team, with over 150 employees successfully trained on the subject.
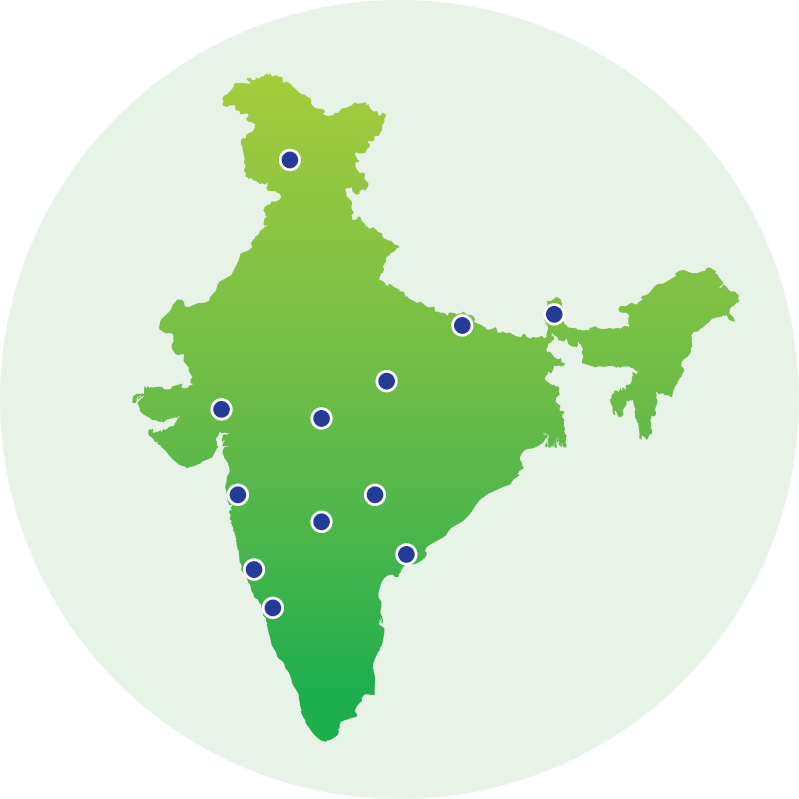
Lupin Sites and Corporate Team
Lupin has partnered with USP India Pvt. Ltd. to conduct training sessions and seminars that strengthen employee understanding of global regulatory standards and best practices. These sessions cover a broad range of topics, including Innovations for the Future of Drug Quality, Handling Customer Complaints (HCC), Understanding Human Errors, and Implementing Corrective and Preventive Action (CAPA). Additional focus areas include Ensuring Good Pharmaceutical Packaging: Extractables and Leachables, USP Education Course - USP <1469> on Nitrosamine Impurities, Corrective and Preventive Actions (CAPA), Managing Residual Solvents, Conducting Deviation Investigations, and Out of Specification Investigations.
Case Study: GTO SPAs
Given the growing need for regulatory compliance, market speed, reliable deliveries, cost optimization, and sustainability, the Global Technical Operations (GTO) has outlined six Strategic Performance Areas (SPAs) with specific objectives, deliverables, and success metrics. Cross functional teams have been deployed with the required capability and resources to deliver on the SPAs.

Quality and Regulatory Compliance
Customer Focus and Growth
Cost Leadership
Technology Leadership
ESG
Capability Building
Way Forward
As we look ahead, our commitment to manufacturing and quality excellence remains unwavering. We will further expand our manufacturing footprint to meet increasing global demand, while simultaneously enhancing our standards with regard to quality, compliance, and efficiency. Our quality and compliance focus is clear, to eliminate product recalls, strengthen inspection readiness, and sustain zero-warning letter status in terms of U.S. FDA audits across all sites.
We are accelerating the integration of automation, digital systems, and AI-enabled platforms across our network. These aren’t just technology upgrades — they are enablers of precision, accountability, and speed. At the same time, we are investing deeply in the upskilling of our workforce. Our people are the true catalysts behind our success, and by eManufacturing Division Upskilmpowering them through holistic training, we ensure that progress is shared and sustainable.
Ultimately, our purpose, to catalyze treatments that transform hope into healing, begins at the shop floor. Every batch we produce, every improvement we implement, is part of this deeper promise. At Lupin, we take pride in transforming lives. And we will continue doing so with care, with courage, and with conviction.