PRODUCTS
| Product Name | Strength | Image (not to scale) | Pack Size | NDC # | RLD / Brand Name | TE Rating | Therapeutic Category |
|---|---|---|---|---|---|---|---|
| Enskyce™ | 0.15 mg /0.03 mg |
 |
28 x 3 |
68180-739-73 |
Ortho-Cept® |
AB |
Oral Contraceptives |
| Ethacrynic Acid Tablets | 25mg |
 |
100 |
68180-0159-01 |
Edecrin® |
AB |
Diuretic |
| Ethambutol Tablets | 100mg |
 |
100 |
68180-280-01 |
Myambutol® |
AB |
Antimycobacterial agent |
| Fenofibrate Capsules | 43mg |
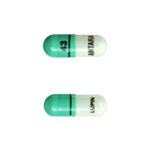 |
30 |
68180-0130-06 |
Antara® |
AB |
Antihyperlipidemic |
| Fenofibrate Tablets | 48mg |
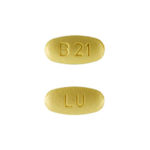 |
90 |
68180-388-09 |
Tricor® |
AB |
Antihyperlipidemic agent |
| Fenofibrate Tablets | 54mg |
 |
90 |
68180-231-09 |
Lofibra® |
AB |
Antihyperlipidemic agent |
| Flucytosine Capsules USP | 250 mg |
 |
100 |
43386-771-01 |
Ancobon® |
AB |
Antifungal |
| Fluocinonide Topical Solution USP | 0.05% |
 |
20 mL |
43386-026-02 |
Lidex® |
AT |
Corticosteroid |
| Fluoxetine Tablets USP | 60mg |
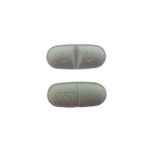 |
30 |
68180-997-06 |
Fluoxetine Tablets |
AB |
Anti-depressant |
| Fluoxetine Tablets USP | 10mg |
 |
30 |
68180-998-01 |
Prozac® |
AB |
Anti-depressant |
| Fosaprepitant Dimeglumine for Injection | 150mg |
 |
5mL single-dose vial |
68180-690-01 |
Emend® |
AP |
Antiemetic |
| Fyavolv™ | 0.5mg/0.0025mg |
 |
28 x 3 |
68180-827-09 |
FemHRT® |
AB |
Hormone Replacement Therapy |
*All registered trademarks are the property of their respective owners. These products are intended for U.S. residents only.
