PRODUCTS
| Product Name | Strength | Image (not to scale) | Pack Size | NDC # | RLD / Brand Name | TE Rating | Therapeutic Category |
|---|---|---|---|---|---|---|---|
| Cefadroxil for Oral Suspension USP | 250 mg/5 mL |
 |
75 mL |
68180-181-02 |
Duricef® |
AB |
Cephalosporin Antibiotic |
| Cefadroxil Capsules USP | 500 mg |
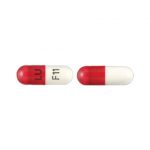 |
50 |
68180-180-08 |
Duricef® |
AB |
Cephalosporin Antibiotic |
| Bupropion Hydrochloride Extended-Release Tablet USP (XL) | 150mg |
 |
30 |
68180-319-06 |
Wellbutrin XL® |
AB |
Antidepressant |
| Bumetanide Injection USP | 1mg/4mL (0.25mg/mL) |
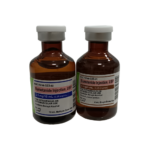 |
(10) 4mL single-dose vial |
70748-323-10 |
Bumex® |
AP |
Loop Diurectic |
| Budesonide Inhalation Suspension | 0.5 mg/2 mL |
 |
30 SD Ampules |
68180-984-30 |
Pulmicort Respules Inhalation Suspension |
AN |
Anti-inflammatory |
| Blisovi™ Fe 1/20 Tablets USP | 1 mg/0.02 mg |
 |
28 x 3 |
68180-865-73 |
Loestrin® Fe 1/20 |
AB |
Oral Contraceptives |
| Blisovi™ Fe 1.5/30 Tablets USP | 1.5 mg/0.03 mg |
 |
28 x 3 |
68180-866-73 |
Loestrin® Fe 1.5/30 |
AB |
Oral Contraceptives |
| Blisovi™ 24 Fe Tablets USP | 1 mg/0.02 mg |
 |
28 x 3 |
68180-864-73 |
Loestrin® 24 Fe |
AB |
Oral Contraceptives |
| Betamethasone Ointment | 0.05% |
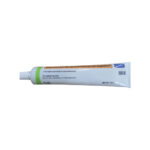 |
50g |
68180-0947-01 |
Diprolene® |
AB |
Anti-inflammatory |
| Azithromycin Tablets USP | 250 mg |
 |
1 x 6 |
68180-861-06 |
Zithromax® |
AB |
Antibiotic |
| Arformoterol Tartrate Inhalation Solution | 15mcg/2mL |
 |
30 UD Vials |
70748-0257-30 |
Brovana® |
NA |
Long-acting beta agonists (LABAs) |
| Amlodipine Besylate Tablets USP | 2.5 mg |
 |
90 |
68180-720-03 |
Norvasc® |
AB |
Calcium Channel Blocker |
*All registered trademarks are the property of their respective owners. These products are intended for U.S. residents only.
