Mumbai, August 03, 2022: Pharma major Lupin Limited [BSE: 500257 | NSE: LUPIN] reported its financial performance for the quarter ending June 30, 2022. These unaudited results were taken on record by the Board of Directors at a meeting held today.
| Financial Highlights – Consolidated IND-AS |
Amt in INR mn
| Particulars | Quarter | ||||
| Q1 FY2023 | Q4 FY2022 | QoQ Growth % | Q1 FY2022 | YoY Growth % | |
| Sales | 36,040 | 38,645 | ↓ 6.7% | 42,374 | ↓ 14.9% |
| EBITDA | 2,379 | 2,823 | ↓ 15.7% | 9,926 | ↓ 76.0% |
| EBITDA Margin (%) | 6.6% | 7.3% | ↓ 70 bps | 23.4% | ↓ 1680 bps |
| PBT | 23 | (852) | ↑102.7% | 7,503 | ↓ 99.7% |
Income Statement highlights – Q1 FY2023
- Gross Profit was INR 19,942 mn compared to INR 22,323 mn in Q4 FY2022, with margin of 55.3%
- Personnel cost was 21.6% of sales at INR 7,785 mn compared to INR 7,032 mn in Q4 FY2022
- Manufacturing and other expenses were 33.1% of sales at INR 11,916 mn compared to INR 13,212 mn in Q4
FY2022 - Investment in R&D for the quarter was INR 3,477.8 mn (9.6% of sales)
Balance Sheet highlights
- Operating working capital was INR 58,983 mn as on June 30, 2022
- Capital Expenditure for the quarter was INR 1,613 mn
- Net Debt as on June 30, 2022 stands at INR 25,143 mn
- Net Debt-Equity for the company as on June 30, 2022 stands at 0.21
Commenting on the results, Mr. Nilesh Gupta, Managing Director, Lupin Limited said, “Our numbers are muted this quarter, but we expect a strong bounce back Q2 onwards. Our U.S. sales took a significant dip as we took several strategic decisions to pave the way for building a sustainable and profitable business. During the quarter we pared down inventories and we took shelf stock adjustments on select products. While we continue to see price erosion in our US business and inflation in input materials, our India business continues to do well growing ahead of the market. All other geographies continue to perform well, and we have now implemented several optimization measures including addressing our workforce in operations and supporting functions to improve our cost position and ensure competitiveness. While the benefits of this optimization will start accruing Q2 onwards, we are now focused on getting back on the growth path driven by growth in key markets like India and multiple important complex generic launches in the US and other developed markets. On the compliance front, we have now satisfactorily addressed both Goa and Somerset, and continue our efforts to be best in class in quality and compliance across our network.”
| Consolidated Financial Results Q1 FY2023 |
Amt in INR mn
| Particulars | Q1 FY2023 | % of sales | Q4 FY2022 | % of sales | QoQ Gr% | Q1 FY2022 | % of sales | YoY Gr% |
| Sales | 36,040 | 100.0% | 38,645 | 100.0% | -6.7% | 42,374 | 100.0% | -14.9% |
| Other operating income | 1,398 | 3.9% | 185 | 0.5% | 655.7% | 328 | 0.8% | 326.2% |
| Total Revenue from operations | 37,438 | 103.9% | 38,830 | 100.5% | -3.6% | 42,702 | 100.8% | -12.3% |
| Material cost | 16,098 | 44.7% | 16,322 | 42.2% | -1.4% | 15,280 | 36.1% | 5.4% |
| Gross Profit (Excl. Other op. income) | 19,942 | 55.3% | 22,323 | 57.8% | -10.7% | 27,094 | 63.9% | -26.4% |
| Employee cost | 7,785 | 21.6% | 7,032 | 18.2% | 10.7% | 7,837 | 18.5% | -0.7% |
| Manufacturing & Other expenses | 11,916 | 33.1% | 13,212 | 34.2% | -9.8% | 10,309 | 24.3% | 15.6% |
| Other Income | 56 | 0.2% | 157 | 0.4% | -64.3% | 278 | 0.7% | -79.9% |
| Forex Loss / (Gain) | (684) | -1.9% | (402) | -1.0% | 70.1% | (372) | -0.9% | 83.9% |
| EBITDA | 2,379 | 6.6% | 2,823 | 7.3% | -15.7% | 9,926 | 23.4% | -76.0% |
| Depreciation, Amortization & Impairment Expense1 | 1,928 | 5.3% | 3,272 | 8.5% | -41.1% | 2,088 | 4.9% | -7.7% |
| EBIT | 451 | 1.3% | (449) | -1.2% | 200.4% | 7,838 | 18.5% | -94.2% |
| Finance cost | 428 | 1.2% | 415 | 1.1% | 3.1% | 335 | 0.8% | 27.8% |
| Adjusted Profit Before Tax (PBT) | 23 | 0.1% | (864) | -2.2% | 102.7% | 7,503 | 17.7% | -99.7% |
| Business Compensation Expense | – | 0.0% | (12) | 0.0% | – | 0.0% | ||
| Profit Before Tax (PBT) | 23 | 0.1% | (852) | -2.2% | 7,503 | 17.7% | ||
| Tax | 891 | 2.5% | 4,267 | 11.0% | 2,023 | 4.8% | ||
| Profit After Tax (PAT) | (868) | -2.4% | (5,119) | -13.2% | 5,480 | 12.9% | ||
| (+) Share of Profit from JV | – | 0.0% | 2 | 0.0% | 2 | 0.0% | ||
| (-) Non-Controlling Interest | 23 | 0.1% | 63 | 0.2% | 57 | 0.1% | ||
| Profit/(Loss) for the period | (891) | -2.5% | (5,180) | -13.4% | 5,425 | 12.8% | ||
- Depreciation, Amortization & impairment expense of Q4 FY22 includes INR 1,267 mn on account of impairment of Gavis IPs.
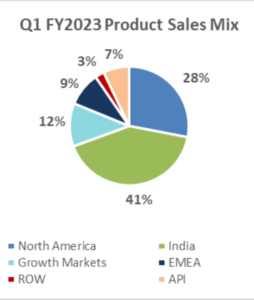
| Sales Mix |
Amt in INR mn
| Particulars | Q1 FY2023 | Q4 FY2022 | Growth QoQ | Q1 FY2022 | Growth YoY |
| North America | 10,104 | 14,162 | -28.7% | 13,330 | -24.2% |
| India | 14,920 | 13,511 | 10.4% | 16,362 | -8.8% |
| Growth Markets | 4,237 | 3,810 | 11.2% | 3,328 | 27.3% |
| EMEA | 3,335 | 4,072 | -18.1% | 2,613 | 27.6% |
| ROW | 893 | 887 | 0.7% | 548 | 63.0% |
| Total Formulations | 33,489 | 36,442 | -8.1% | 36,181 | -7.4% |
| API | 2,551 | 2,203 | 15.8% | 2,459 | 3.7% |
| Total Product Sales | 36,040 | 38,645 | -6.7% | 38,640 | -6.7% |
| NCE Licensing Income | NA | NA | 3,734 | ||
| Consolidated Sales | 36,040 | 38,645 | -6.7% | 42,374 | -14.9% |
| Operational Highlights |
North America
North America sales for Q1 FY2023 were INR 10,104 mn, down 28.7% compared to INR 14,162 mn in Q4 FY2022; down 24.2% as compared to INR 13,330 mn in Q1 FY2022; accounting for 28% of Lupin’s global sales.
Q1 FY2023 sales were USD 121 mn compared to USD 181 mn in Q4 FY2022 and USD 172 mn in Q1 FY2022.
The Company filed 4 ANDAs in the quarter, received 4 ANDA approvals from the U.S. FDA, and launched 1 product in the quarter in the U.S. The Company now has 167 generic products in the U.S.
Lupin continues to be the 3rd largest pharmaceutical player in both U.S. generic market and U.S.
total market by prescriptions (IQVIA MAT June 2022). Lupin is the leader in 44 of its marketed generics in the
U.S. and amongst the Top 3 in 113 of its marketed products (IQVIA MAT March 2022).
India
India formulation sales for Q1 FY2023 were INR 14,920 mn, up 10.4% as compared to INR 13,511 mn in Q4 FY2022; down 8.8% as compared to INR 16,362 mn in Q1 FY2022; accounting for 41% of Lupin’s global sales.
India Region Formulations sales grew by 9.9% in the quarter as compared to Q4 FY2022, down 2.9% as compared to Q1FY2022. The company launched 6 brands across therapies during the quarter.
Lupin is the 6th largest company in the Indian Pharmaceutical Market (IQVIA MAT June 2022).
Growth Markets (LATAM and APAC)
Growth Markets registered sales of INR 4,237 mn for Q1 FY2023, up 11.2% compared to INR 3,810 mn in Q4 FY2022; up 27.3% as compared to INR 3,328 mn in Q1 FY2022; accounting for 12% of Lupin’s global sales.
Brazil sales were BRL 57 mn for Q1 FY2023, compared to BRL 64 mn for Q4 FY2022 and BRL 63 mn for Q1 FY2022.
Mexico sales were MXN 213 mn for Q1 FY2023, compared to MXN 183 mn for Q4 FY2022 and MXN 163 mn for Q1 FY2022.
Philippines sales were PHP 434 mn for Q1 FY2023, compared to PHP 475 mn for Q4 FY2022 and PHP 362 mn for Q1 FY2022.
Australia sales were AUD 25.2 mn for Q1 FY2023, compared to AUD 20.8 mn for Q4 FY2022 and AUD 17.1 mn for Q1 FY2022.
Europe, Middle-East and Africa (EMEA)
EMEA sales for Q1 FY2023 were INR 3,335 mn, down 18.1% compared to INR 4,072 mn in Q4 FY2022; up 27.6% compared to INR 2,613 mn in Q1 FY2022; accounting for 9% of Lupin’s global sales.
South Africa sales were ZAR 282 mn for Q1 FY2023, compared to ZAR 426 mn for Q4 FY2022 and ZAR 273 mn for Q1 FY2022. Lupin is the 6th largest player in South Africa in the total generics market (IQVIA May
2022).
Germany sales were EUR 9.2 mn for Q1 FY2023, compared to EUR 8.4 mn for Q4 FY2022 and EUR 7.4 mn for Q1 FY2022.
Global API
Global API Sales for Q1 FY2023 were INR 2,551 mn, up 15.8% as compared to INR 2,203 mn in Q4 FY2022; up 3.7% as compared to INR 2,459 mn in Q1 FY2022; accounting for 7% of Lupin’s global sales.
Research and Development
Investment in R&D was INR 3,477.8 mn (9.6% of sales) for Q1 FY2023 as compared to INR 3442 mn (8.9% of
sales) for Q4 FY2022.
Lupin received approval for 4 ANDAs from the U.S. FDA in the quarter. Cumulative ANDA filings with the U.S. FDA stand at 459 as of June 30, 2022, with the company having received 301 approvals to date.
The Company now has 54 First-to-File (FTF) filings including 21 exclusive FTF opportunities. Cumulative U.S. DMF filings stand at 173 as of June 30, 2022.
About Lupin
Lupin is an innovation-led transnational pharmaceutical company headquartered in Mumbai, India. The Company develops and commercializes a wide range of branded and generic formulations, biotechnology products, and APIs in over 100 markets in the U.S., India, South Africa, and across the Asia Pacific (APAC), Latin America (LATAM), Europe, and Middle East regions.
The Company enjoys a leadership position in the cardiovascular, anti-diabetic, and respiratory segments and has
a significant presence in the anti-infective, gastro-intestinal (GI), central nervous system (CNS), and women’s
health areas. Lupin is the third-largest pharmaceutical company in the U.S. by prescriptions. The company
invested 8.7% of its revenue in research and development in FY22.
Lupin has 15 manufacturing sites, 7 research centers, more than 20,000 professionals working globally, and has
been consistently recognized as a ‘Great Place to Work’ in the Biotechnology & Pharmaceuticals sector.
Please visit www.lupin.com for more information.
Follow us on Twitter: https://twitter.com/LupinGlobal
LinkedIn : https://www.linkedin.com/company/lupin
Facebook: http://www.facebook.com/LupinWorld/
For further information or queries please contact –
Shweta Munjal
Vice President & Global Head – Corporate Communications
Email: shwetamunjal@lupin.com